Effects of Halophilic Bacteria's Polyunsaturated Fatty Acids (PUFAs) on Human Health
DOI:
https://doi.org/10.31033/abjar.2.1.3Keywords:
polyunsaturated, production, human health, fish, halophilic bacteria, plantsAbstract
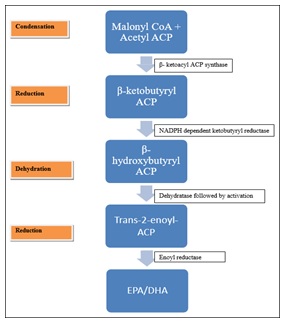
The need for polyunsaturated fatty acid (PUFA) generation from fish oil and plants has increased with the rise in health consciousness. PUFAs are extremely advantageous for human health and have a positive influence on heart and brain function. Humans cannot generate PUFAs on their own because they are essential fatty acids, so they must get them from their food. PUFAs are long-chain hydrocarbons with multiple double bonds that have methyl and carboxyl groups at opposite ends. The focus is mostly on halophiles due to the desire for PUFAs that can be produced at low cost, practically, and without risk, as well as the problem of contaminated fish oil and the need to prevent the exploitation of plants and marine life. High-salinity environments are ideal for halophilic bacteria. They are capable of withstanding salt concentrations of up to 30% and 1.7% (0.3 m) (5.1 m). Halophiles are effective at producing PUFAs on a large scale. In addition to resolving the problem of fish-derived PUFAs, which is a problem for the majority of vegans, switching from fish-derived PUFAs to microbial PUFAs has the potential to be a revolutionary and entirely sustainable solution. Eicosapentaenoic acids (EPA) and docosahexaenoic acids (DHA), which are obtained from halophilic bacteria, will be produced, characterized, and discussed in this article along with their comparison to PUFAs derived from fish and plants.
Downloads
References
Allen EE, & Bartlett DH. (2002). Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology, 148(6), 1903–1913.
Alonso DL, & Maroto FG. (2000). Plants as ‘chemical factories’ for the production of polyunsaturated fatty acids. Biotechnology Advances, 18(6):481–497. https://doi.org/https://doi.org/10.1016/S0734-9750(00)00048-3.
Bowman JP, McCammon SA, Nichols DS, Skerratt JH, Rea SM, & Nichols PD et al. (1997). Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5 omega 3) and grow anaerobically by dissimilatory Fe(III) reduction. International Journal of Systematic Bacteriology, 47(4), 1040–1047. https://doi.org/10.1099/00207713-47-4-1040.
Bowman John P, Gosink JJ, McCAMMON SA, Lewis TE, Nichols DS, & Nichols PD et al. (1998). Colwellia demingiae sp. nov., Colwellia hornerae sp. nov., Colwellia rossensis sp. nov. and Colwellia psychrotropica sp. nov.: psychrophilic Antarctic species with the ability to synthesize docosahexaenoic acid (22: ω63). International Journal of Systematic and Evolutionary Microbiology, 48(4), 1171–1180.
Cao Y, & Cao Y. (2012). Biotechnological production of eicosapentaenoic acid: from a metabolic engineering point of view. Process Biochemistry, 47(9), 1320– 1326.
Freese E, Rütters H, Köster J, Rullkötter J, & Sass H. (2009). Gammaproteobacteria as a Possible Source of Eicosapentaenoic Acid in Anoxic Intertidal Sediments. Microbial Ecology, 57(3), 444–454. https://doi.org/10.1007/s00248-008-9443-2.
Gong Y, Wan X, Jiang M, Hu C, Hu H, & Huang F. (2014). Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Progress in Lipid Research, 56, 19–35.
Hamamoto T, Takata N, Kudo T, & Horikoshi K. (1995). Characteristic presence of polyunsaturated fatty acids in marine psychrophilic vibrios. FEMS Microbiology Letters, 129(1), 51–56. https://doi.org/https://doi.org/10.1016/0378-1097 (95)00134-Q.
Jadhav VV, Jamle MM, Pawar PD, Devare MN, & Bhadekar RK. (2010). Fatty acid profiles of PUFA producing Antarctic bacteria: correlation with RAPD analysis. Annals of Microbiology, 60(4), 693–699. https://doi.org/10.1007/s13213-010-0114-4.
Kawamoto J, Kurihara T, Yamamoto K, Nagayasu M, Tani Y, & Mihara H et al. (2009). Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. Journal of Bacteriology, 191(2), 632–640.
Moi IM, Leow ATC, Ali MSM, Rahman RNZRA, Salleh AB, & Sabri S. (2018). Polyunsaturated fatty acids in marine bacteria and strategies to enhance their production. Applied Microbiology and Biotechnology, 102(14), 5811–5826.
Nogi Y, Masui N, & Kato C. (1998). Photobacterium profundum sp. nov, a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles : Life under Extreme Conditions, 2(1), 1–7. https://doi.org/10.1007/s007920050036.
Nogi Yuichi, Kato C, & Horikoshi K. (1998). Moritella japonica sp. nov., a novel barophilic bacterium isolated from a Japan Trench sediment. The Journal of General and Applied Microbiology, 44(4), 289–295.
Orikasa Y, Nishida T, Yamada A, Yu R, Watanabe K, & Hase A et al. (2006). Recombinant production of docosahexaenoic acid in a polyketide biosynthesis mode in Escherichia coli. Biotechnology Letters, 28(22), 1841–1847.
Ratledge C. (204). Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie, 86(11), 807–815.
Downloads
Published
How to Cite
Issue
Section
ARK
License
Copyright (c) 2023 Gaurabh Maniratnam

This work is licensed under a Creative Commons Attribution 4.0 International License.
Research Articles in 'Applied Science and Biotechnology Journal for Advanced Research' are Open Access articles published under the Creative Commons CC BY License Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/. This license allows you to share – copy and redistribute the material in any medium or format. Adapt – remix, transform, and build upon the material for any purpose, even commercially.










