Transcription Protease Synthase Discovered by Strain Solibacillus Sylvestris
DOI:
https://doi.org/10.31033/abjar.2.2.4Keywords:
alkaline protease, Solibacillus silvestris, Sylvestris, salinity variation, environmentsAbstract
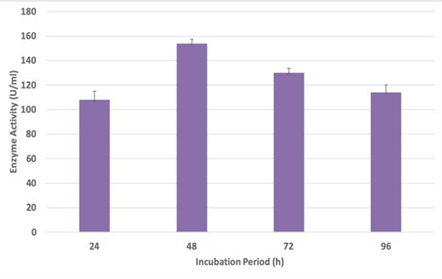
The goal of this work is to maximise the synthesis of bacterial alkaline protease from a mangrove isolate, Solibacillus silvestris, which has the capacity to create valuable primary and secondary metabolites of biotechnological value. Methodology: Using MALDI-TOF (mass spectrometry), the bacterium (known as Madhwad 103 Summer Zobell) used in the current study was isolated from soil that was obtained from Madhwad, Gir, India, during the summer. It was subsequently examined, utilising various physical and chemical variables, since it exhibited the greatest alkaline protease enzyme activity of the isolates. Selected parameters, i.e., Response Surface Methodology (RSM), were also applied to the optimisation of sucrose, peptone, and salinity. Results: Solibacillus silvestris was identified as the bacterium, which was given the name Madhwad 10-3 Summer Zobell. The results showed that the highest enzyme production can be achieved at pH 8.5, 40 °C of temperature, 48 hours of incubation, and an agitation speed of 120 rpm, while the highest enzyme activity was found to be given by sucrose and peptone with 0% salinity, with values of 221.7 U/ml and 246.7 U/ml, respectively. All the factors were significant in the statistical analysis carried out using RSM, which produced the maximum result of 258.750 U/ml at 2.5% sucrose, 10% peptone, and 0% salinity. Applications and improvements: The enzyme created as a result of its effectiveness at operating at an alkaline pH can be used in bioremediation as well as the detergent and textile industries.
Downloads
References
Aguilar JG dos S, de Castro RJ, & Sato HH. (2019). Alkaline protease production by Bacillus licheniformis LBA 46 in a bench reactor: Effect of temperature and agitation. Brazilian Journal of Chemical Engineering, 36(2), 615–625. https://dx.doi.org/10.1590/0104-6632.20190362s20180014
Sharma KM, Kumar R, Panwar S, & Kumar A. (2017). Microbial alkaline proteases: Optimization of production parameters and their properties. Journal of Genetic Engineering and Biotechnology, 15(1), 115–126. https://doi.org/10.1016/j.jgeb.2017.02.001
Ambrose, S.A., Bridges, M.W., DiPietro, M., Lovett, M.C., & Norman, M. K. (2015). How learning works: Seven research-based principles for smart teaching. San Francisco: Jossey-Bass.
Rao MB, Tanksale AM, Ghatge MS, & Deshpande VV. (1998). Molecular and biotechnological aspects of microbial proteases. Microbiol and Molecular Biology Review, 62(3), 597–635. https:// mmbr.asm.org/content/62/3/597.
Saggu SK, & Mishra PC. (2017). Characterization of thermostable alkaline proteases from Bacillus infantis SKS1 isolated from garden soil. PLoS One, 12(11), e0188724. https://10.1371/journal. pone.0188724. eCollection 2017.
Kalwasińska A, Jankiewicz U, Felföldi T, Burkowska-But A, & Brzezinska MS. (2018). Alkaline and halophilic protease production by Bacillus luteus H11 and its potential industrial applications. Food Technology and Biotechnology, 56(4), 553. https://10.17113/ftb.56.04.18.5553.
Indian Coal and Lignite Resources. (2018). Geological Survey of India.
Many DA, Ibrahim AS, & El-Diwany AI. (2019). Biosynthesis of alkaline protease by alkaliphilic Bacillus sp. NPST-AK15 cells immobilized in gel matrices. Egyptian Pharmaceutical Journal. 18(3), 201–207. https://10.4103/epj.epj_2_19.
Downloads
Published
How to Cite
Issue
Section
ARK
License
Copyright (c) 2023 Gaurav Singh, Naveen Srivastava

This work is licensed under a Creative Commons Attribution 4.0 International License.
Research Articles in 'Applied Science and Biotechnology Journal for Advanced Research' are Open Access articles published under the Creative Commons CC BY License Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/. This license allows you to share – copy and redistribute the material in any medium or format. Adapt – remix, transform, and build upon the material for any purpose, even commercially.










